Age of the Earth Series - 3
Lecture 3 - Creationist Arguments Against Radiometric Dating Techniques
In this lecture, the third in my series of lectures on the age of the earth, I intend to highlight the most common arguments I come across in creationist literature and from creationists themselves against radiometric dating techniques. I will use the information from the first two lectures to show why each and every one of these is false. Then in my next lecture, I'll show why all the supposed proofs of a young earth are also false.
Clearly I won't be able to cover every single argument in either this lecture or the next. I cannot claim to have read every single piece of creationist propaganda ever. But I'm pretty experienced in this field, having had many debates with many creationists, and having read many creationist texts and websites. So I hope to give a good insight into their methods and arguments, and strong rebuttals for them all.
Argument 1: You have to know the initial conditions of a rock to date it, including how much parent and daughter were in a rock. This is impossible, because noone was there when it formed
Well, now we've heard the first two lectures, this is a simple argument to answer. Even the simplest Uranium-Lead methods use minerals that exclude lead chemically, and thereby excluding the possibility of non-radiogenic lead being present within the rock at formation.
K-Ar dating uses the fact that Argon is an inert gas and will bubble away at formation - any atmospheric argon-40 that is trapped at formation will also be trapped with atmospheric Argon-36, in a ratio of 295 parts to 1 (the atmospheric ratio of Ar-40 to Ar-36). We can therefore measure the Argon-36 in a sample and work out how much Argon-40 is non-radiogenic, and remove this from our calculations. Since the K-Ar method uses potassium salts, which are hard and crystalline in a strong ionically bonded lattice, we can be sure that no Potassium-40 has been lost or added since formation.
In isochron methods, we need no information whatsoever about the starting concentrations of elements, only the gradient of the isochron. In fact, as we have seen, even if we did need these starting ammounts, the y-intercept of the isochron gives us these directly - we can actually measure the starting ammounts using the isochron method.
Argument 2: Radiometric dating assumes closed system behaviour - that nothing leaks out or gets into a sample post-formation. Therefore any open system behaviour will make radiometric dating unreliable.
Again, this is simply not true. As we know from isochron methods, the isochron will tell us if open system behaviour has occured - because it will not be a straight line. Therefore we cannot get a "false" date from an isochron without knowing it.
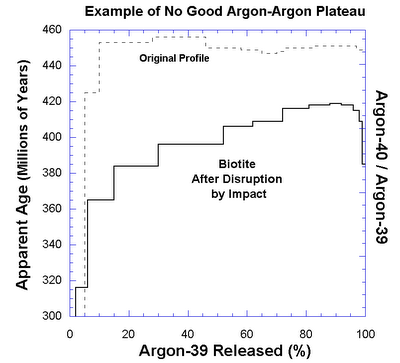
Furthermore, the Ar-Ar methods and the Concordia/Discordia method both can deal with open system behaviour - in fact the latter is *designed* to deal with such behaviour. A discordia cannot exist unless there has been open system behaviour!
Argument 3: Radiometric dating of samples often gives different ages for different methods
Actually, this is just false - the power and strength of radiometric dating is that, as we have shown in lecture 2, we often get massive levels of agreement from many different methods of dating. Of course, given the hundreds of thousands of dating samples now taken, there are bound to be some that will give discordant readings - a mixing isochron here and there - a K-Ar date disrupted by open-system behaviour - and inherited isochron.
But the bulk majority of radiometric dating samples do come back with similar dates over many different and independent techniques. There is now more than sufficient evidence from these to conclude with a high degree of certainty that the earth is old.
Argument 4: Carbon-14 dating is reliant on calibration, you have to know how much C-14 was in the atmosphere at any one time, and you can't know that.
Despite the fact that Carbon-14 dating has never been used by any credible scientist to date the age of the earth - and despite the fact that most biblical archaeology relies on C-14 dating, I see this argument an awful lot.
Firstly, you'll have noted from lectures 1 and 2 that I didn't mention C-14 dating. That's because it is never used to date the age of the earth. This is for two reasons - firstly that it can only date things up to 55,000 years old, because of the short half life of C-14 - and the earth is 4.5 billion years old. Secondly, it does need objects of known age to calibrate it - the creationist argument is partially right - we already have to know that the earth is 50,000 years old because we have to have an object known to be 50,000 years old to make C-14 work.
However, secondly, we do have such objects. We find out the ammount of C-14 in the atmosphere at any one time in the past 50,000 years using 3 methods. Firstly, dendochronology - the study of tree rings. Secondly, varves, seasonal deposition of sediment in sea beds that give a year by year record of the earth's atmosphere going back tens of thousands of years. Thirdly, stalactites and stalagmites are useful to cross check the former two methods.
Argument 5: It is impossible to make accurate measurements of half life - especially for long lived isotopes like Rb-87, whose supposed half life is 47 billion years. Has anyone been around that long to check it?
Half life is actually measured from the activity of a sample (how many decays there are per second from a known quantity of sample), not from waiting around 47 billion years to see if half has decayed.
Basically, in order to establish the half-life of an element, the only thing we need to know is: what is the probability of any one atom decaying in any one second? What that means is, if we take a large enough sample with enough atoms in it (a sample of 1 gram of Rb-87 will contain 7 thousand billion billion atoms), and measure how many atoms decay for a long enough time, we will be able to work out the probability of any one atom decaying in any one second.
From this we can very accurately establish the half life, sufficiently accurately for the "error" in our estimation of half life to be negligable compared to random statistical errors in methods of dating.
Argument 6: But what if half life has changed? There is no way of going back into the past and measuring it?
Having had the previous 5 arguments answered a million times, creationists are now getting desperate. They know fine well that many methods of radiometric dating agree - and they know they are very well tested. So they are trying to come up with something that could be more fundamentally wrong with radiometric dating, in this case, the idea that during the year of Noah's Flood - the half life of elements changed such that 4.5 billion years of decay occured in a single year.
Methods of measuring decay rates in the past:
There are, as you can expect, many problems with this assertion. The first problem is that it is actually possible to measure half life in the past through several methods. The first method is to use a time machine, and look into the past. By "time machine" I mean "telescope". You see, if you look out of your window and look at the sun, you're not seeing the sun right now, you're seeing it 8 minutes ago. That's how long the light has taken to reach you. If I look at a sun that is very far away, say 4000 light years, I will see 4000 years into the past. We have studied stars hundreds of thousands of light years away - and we have measured decay of radioactive elements within those stars.
Another method of reassuring ourselves that decay rates have not changed is by looking at Uranium decay chains / series. I mentioned these in lecture 1. You see, these chains settle down to a steady state - called a "secular equilibrium" - where the ammounts of each element in the chain remain the same. The ratio of each element to each other element at secular equilibrium is determined by the ratio of their half-lives. What that means is that, if half lives had changed say, 4000 years ago, every decay chain would have been disturbed. Then if the half-lives had changed back to present day values say, 3999 years ago, it would take another 2-3 million years for these chains to go back into secular equilibrium. In other words, if this creationist assertion were true, we should find a total of zero uranium decay chains at secular equilibrium. In fact, we find many.
There are other methods of measuring decay rates in the past, although they are more complex. For example, the neutron absorbtion ratio from natural nuclear reactors such as the Oklo reactor can be used to measure physical constants and decay rates in the long past. But discussion of these sorts of methods is outwith the scope of this lecture, enthusiasts can write to me to find out.
Other problems with half-life dramatically changing:
There are several other problems with the idea that half-life has dramatically changed. Firstly, as we learnt in lecture 1, we have a good understanding of the theory behind nuclear physics, and what causes a nucleus to be radioactive. We know that radical change to half-life would involve radical change to the constants in the Semi-empirical mass formula. There is no reason at all why this should occur just because there was a worldwide flood. Not only that, God would have to arrange it such that all elements had the same percentage change in decay rate so that radiometric dates would agree - how or why he would do so is open to question. What physical constants he might alter to do this are unknown.
However, there is a much bigger problem with the idea of half life changing dramatically, and that problem is heat. We learnt in lecture 1 that the reason nuclei decayed is that they were unstable - they had too much energy. The Semi-empirical mass formula is actually a measure of the energy each nucleus has. In other words, radioactive decay happens because nuclei have too much energy, they are unstable, and they need to get rid of some energy to become more stable.
What that means is that all radioactive decay produces heat. There are no exceptions to this rule. If it doesn't release energy, it isn't radioactive decay. In fact, radioactivity produces a lot of heat. We all know this - because we all know what happens when you make unstable nuclei like Uranium split apart in a process called fission (thus releasing the energy of several decays at once). You get a big explosion. Hiroshima style. Or you get a nuclear power plant. In fact, the reason the core of the earth is still hot, despite it loosing heat, is because of natural radioactivity keeping it hot.
Now creationists want 4.5 billion years worth of decay to have happened in a year. That means an increase in radioactivity of about 450 billion percent. Sure, the clever ones among you are saying "does all that energy mean the rocks would melt and isochrons would be reset and Argon would be released?" And yes, I guess it does. But more worryingly, it means that large parts of the earth would be vapourised or melted. That obviously hasn't happened in the last 4000 years. Heat is a more general problem for "flood geology" actually - what with the heat produced from all the continents shifting at several meters per second, and of course the idea that almost all the igneous rock was produced in that time.
Argument 7: What about pressure and heat - do these not affect half-life and make radiometric dating inaccurate?
The answer to this is generally no. We have subjected light elements to hundreds of thousands of atmospheres of pressure and pretty extreme heat, and we have only seen minute changes to radioactive decay. The reason for this is that the nucleus where decay occurs is pretty immune to either chemical environment or outside pressure. It is not involved in chemical bonding, and the forces within it are so powerful as to render even massive external pressure negligable.
The only exception to this is for bound-state beta decay in a plasma. Let me explain. Beta-decay is when a neutron turns into a proton and an electron. In bound state beta decay, this electron does not escape, but actually starts orbiting the new nucleus. Such decay is common in Rhenium-187 in the interior of stars, where the heat from the stars has stripped many or all of the outer electrons of the Rhenium, and therefore bound state beta decay can occur.
In other words, when beta-decaying elements exist in a plasma state, with their outer electrons ripped away, beta-decay can happen much more quickly in a "bound-state" form. One young-Earth proponent suggested that God used plasma conditions when He created the Earth a few thousand years ago. This writer suggested that the rapid decay rate of rhenium under extreme plasma conditions might explain why rocks give very old ages instead of a young-Earth age. This writer neglected a number of things, including: a) plasmas only affect a few of the dating methods. More importantly, b) rocks and hot gaseous plasmas are completely incompatible forms of matter!
Argument 8: What if God made the earth mature, like he made Adam mature, and created the star-light in transit?
This final question intrudes slightly on the topic of Lecture 5 - the theology of an old earth, but it is worth dealing with here. Most people who are ignorant of the evidence for an old earth, and ignorant of the science of cosmology, advance this kind of argument because they don't think through the actual implications.
When we look up at the stars we just see points of light. They're pretty. We can lie back on the grass with our girlfriends and gaze up at them, and hope that the romance of the situation will lead to a snog and a grope. It is easier to believe that God might just have created the light in transit to give us a such a great view, and a better chance at a snog and a grope.
But when scientists look at stars, they don't just see points of light. They look through telescopes, and see all sorts of wonderful details - but the most important detail of all is that these stars are not static. Stuff happens in them. Events. Things are going on. From radioactive decay, to cosmic jets, to supernovae expanding and contracting - these stars are a hotbed of activity! If God created the light in transit therefore - there is only one conclusion we can make about all this activity - all of these events we see. They never happened.
God made them up, like Steven Speilberg makes a CGI movie sequence up. If we observe a star going nova 500,000 light years away - the supernova we see forming never formed. That star might not actually exist, for all we know, because we have never really seen it. All of the decays we counted, all of the expansion we measured, all of the flashes and movement we saw - was all an elaborate hoax - a movie reel - a piece of divine CGI animation.
All the chicks love a scar
We all build up scars in our lives, and stretch marks, and various other annoyingly permanent but occasionally impressive blemishes. They remind us of how we became who we are - of the period where we lost weight quickly - that time we fell off our bikes when we were 6 - the tooth we chipped playing rugby. Scars are permanent reminders of real incidents in our past - our life history is written in them - we weren't created yesterday.
Sadly, there are still people around whose minds have been so bent and twisted by religious fundamentalism, that they believe the earth *was* created yesterday. Or, more specifically, about 6,000 years ago. They say that, if God created Adam mature, why should He not create the earth mature? But I have news for them. If God created Adam, he didn't create him with a scar on his navel from an operation on his gall bladder. Nor would Adam have possessed scarring from a bilateral hernia, nor a BCG injection scar. His teeth would have been perfect and new, and showed no signs of the wear and tear they would have gone through in teenage years, because he didn't have teenage years. His little toes would never have been broken, because he'd never broken them. He would have had no stretch marks, because he would never have grown. Adam didn't have a history, and God isn't a liar.
The earth has so many scars. Of great meteor impacts that eradicated dinosaurs in the Cretaceous, and rocked the end-Permian era. Of animal tracks buried under layers of sediment, built up over millions of years. The fossils of beings the likes of which we've never seen, the radioactive elements in the rocks that contain them, the order of the strata in which the rocks lie - ghosts of a past, a real history, that haunt the modern day creationist. And even when we turn our gaze away from the earth beneath us towards the heavens, using our telescopes to see stars hundreds of thousands of light years away, are we to seriously believe that we observe a faked history? Are we to imagine that the God of truth created images of supernovae exploding, and coded these images into light, and put that light in transit toward earth - even though the supernovae never existed - and the explosions never occurred?
And I'll let you ponder those questions for a little while longer, as I create lecture 4 in this series!